The global laboratory proficiency testing (PT) market was valued at an estimated USD 1.36 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.6% from 2024 to 2030. Several key factors are expected to drive this market expansion, including an increased emphasis on water testing, the legalization of medical cannabis, a rising number of cannabis testing laboratories, a growing incidence of foodborne illnesses, an uptick in chemical contamination cases in food, and the ongoing introduction of new products and services. For example, in March 2022, BIPEA launched an innovative Proficiency Testing Scheme (PT 35d) specifically designed for laboratories focused on water microbiological testing. Additionally, the heightened adoption of laboratory PT due to stringent regulatory requirements is anticipated to further fuel market growth in the years ahead.
In the context of water testing, laboratory PT for endotoxin and pyrogen levels is particularly vital. Endotoxins are toxic substances released from the outer membrane of specific bacteria, while pyrogens are compounds that can induce fever and other negative health effects. Proficiency testing ensures that laboratories can accurately measure and evaluate endotoxin and pyrogen levels in water samples. It also validates the laboratory's ability to employ appropriate testing methodologies, such as Limulus Amebocyte Lysate (LAL) assays, for detecting and quantifying these contaminants. Accurate testing for endotoxins and pyrogens is essential for assessing the safety and quality of water, particularly in industries such as pharmaceutical production, medical device manufacturing, and dialysis. Proficiency testing in this domain guarantees reliable and consistent results, facilitating informed decisions that protect public health and ensure regulatory compliance in water-related sectors.
Gather more insights about the market drivers, restrains and growth of the Laboratory Proficiency Testing Market
Industry Insights
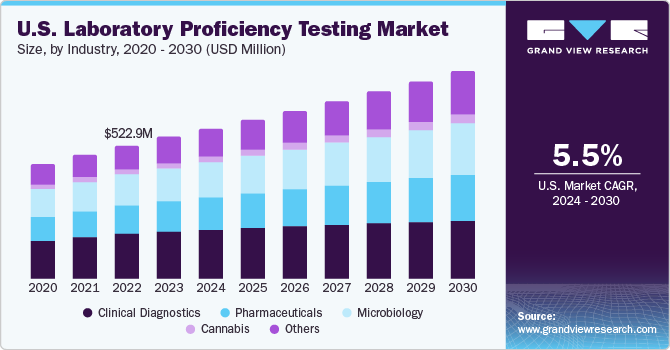
The clinical diagnostic segment accounted for the largest share of the market, holding 33.32% in 2023. Clinical diagnostic laboratories generally exhibit a lower likelihood of diagnostic errors compared to hospitals, making proficiency testing a widely adopted practice to maintain accurate results and effective quality management. Acknowledging the importance of PT, the Centers for Medicare and Medicaid Services (CMS) proposed updates in July 2022 to the requirements for laboratories regulated under the Clinical Laboratory Improvement Amendments (CLIA). These updates aim to enforce stricter standards, thereby enhancing the accuracy and reliability of laboratory testing and ensuring that CLIA laboratories adhere to the highest quality benchmarks.
The cannabis segment is expected to experience the fastest CAGR during the forecast period. With the expansion of cannabis legalization, there has been a significant increase in the demand for accurate and reliable testing. Laboratory proficiency testing plays a crucial role in validating the competence and accuracy of cannabis testing laboratories, enabling them to meet regulatory standards and provide consumers with assurance regarding the quality and safety of cannabis products. For instance, in April 2023, Trust in Testing introduced national standards for cannabis testing, aimed at improving the reliability and credibility of testing practices across the industry.
Order a free sample PDF of the Laboratory Proficiency Testing Market Intelligence Study, published by Grand View Research.