Protein Engineering: Solving Biological Challenges
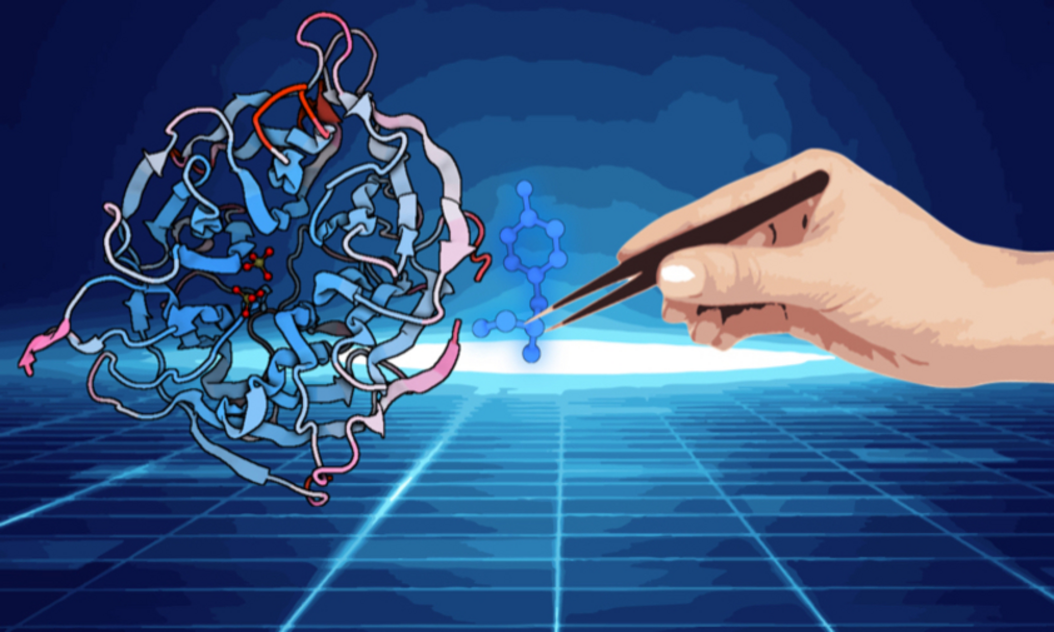
Protein engineering is at the forefront of modern biotechnology, offering the potential to create custom-designed proteins with specific, desirable properties that can revolutionize various fields, from medicine to agriculture and environmental sustainability. This scientific discipline involves the modification or creation of proteins through methods like directed evolution and rational design. By altering the amino acid sequences, researchers can enhance a protein's functionality, stability, and specificity, tailoring it to meet specific needs and applications.
One of the most significant applications of protein engineering is in the development of therapeutic proteins and enzymes. These engineered proteins can be used to treat a wide range of diseases, including cancer, diabetes, and genetic disorders. For example, monoclonal antibodies, which are proteins engineered to target specific antigens, have become a cornerstone in cancer treatment, offering precise targeting of cancer cells while minimizing damage to healthy tissue. Similarly, insulin analogs created through protein engineering have improved the management of diabetes by offering more predictable absorption and longer-lasting effects.
In addition to therapeutic applications, protein engineering is revolutionizing industrial processes. Enzymes engineered to be more stable and efficient can replace harsh chemicals in manufacturing processes, leading to more sustainable and eco-friendly production methods. For instance, engineered enzymes are used in the production of biofuels, where they help break down biomass into fermentable sugars more efficiently than natural enzymes. This not only reduces the cost of biofuel production but also minimizes the environmental impact.
Agriculture is another sector benefiting from protein engineering. By designing proteins that can enhance crop resistance to pests, diseases, and harsh environmental conditions, scientists are contributing to more sustainable agricultural practices. For example, Bt toxins, engineered proteins derived from the bacterium Bacillus thuringiensis, have been incorporated into genetically modified crops to provide resistance against specific insect pests, reducing the need for chemical pesticides.
The environmental impact of protein engineering is also significant. Engineered proteins can be designed to degrade pollutants, offering potential solutions for environmental cleanup efforts. For example, enzymes capable of breaking down plastic waste or toxic pollutants into harmless compounds are being developed, providing innovative approaches to address pressing environmental challenges.
Despite the tremendous potential, protein engineering faces challenges, including the complexity of protein structures and functions, and the need for advanced computational tools to predict and design protein modifications accurately. However, ongoing advancements in computational biology and machine learning are addressing these challenges, making protein engineering more precise and efficient.
In summary, protein engineering represents a transformative approach to biotechnology, with applications that span medicine, industry, agriculture, and environmental sustainability. By designing proteins with enhanced or novel functions, scientists are pushing the boundaries of what is possible, leading to innovations that have the potential to improve human health, protect the environment, and drive economic growth. As research and technology continue to advance, the impact of protein engineering is set to grow, heralding a new era of scientific and technological breakthroughs.
https://www.marketdigits.com/protein-engineering-market-1704808374 Protein Engineering: Solving Biological Challenges
Protein engineering is at the forefront of modern biotechnology, offering the potential to create custom-designed proteins with specific, desirable properties that can revolutionize various fields, from medicine to agriculture and environmental sustainability. This scientific discipline involves the modification or creation of proteins through methods like directed evolution and rational design. By altering the amino acid sequences, researchers can enhance a protein's functionality, stability, and specificity, tailoring it to meet specific needs and applications.
One of the most significant applications of protein engineering is in the development of therapeutic proteins and enzymes. These engineered proteins can be used to treat a wide range of diseases, including cancer, diabetes, and genetic disorders. For example, monoclonal antibodies, which are proteins engineered to target specific antigens, have become a cornerstone in cancer treatment, offering precise targeting of cancer cells while minimizing damage to healthy tissue. Similarly, insulin analogs created through protein engineering have improved the management of diabetes by offering more predictable absorption and longer-lasting effects.
In addition to therapeutic applications, protein engineering is revolutionizing industrial processes. Enzymes engineered to be more stable and efficient can replace harsh chemicals in manufacturing processes, leading to more sustainable and eco-friendly production methods. For instance, engineered enzymes are used in the production of biofuels, where they help break down biomass into fermentable sugars more efficiently than natural enzymes. This not only reduces the cost of biofuel production but also minimizes the environmental impact.
Agriculture is another sector benefiting from protein engineering. By designing proteins that can enhance crop resistance to pests, diseases, and harsh environmental conditions, scientists are contributing to more sustainable agricultural practices. For example, Bt toxins, engineered proteins derived from the bacterium Bacillus thuringiensis, have been incorporated into genetically modified crops to provide resistance against specific insect pests, reducing the need for chemical pesticides.
The environmental impact of protein engineering is also significant. Engineered proteins can be designed to degrade pollutants, offering potential solutions for environmental cleanup efforts. For example, enzymes capable of breaking down plastic waste or toxic pollutants into harmless compounds are being developed, providing innovative approaches to address pressing environmental challenges.
Despite the tremendous potential, protein engineering faces challenges, including the complexity of protein structures and functions, and the need for advanced computational tools to predict and design protein modifications accurately. However, ongoing advancements in computational biology and machine learning are addressing these challenges, making protein engineering more precise and efficient.
In summary, protein engineering represents a transformative approach to biotechnology, with applications that span medicine, industry, agriculture, and environmental sustainability. By designing proteins with enhanced or novel functions, scientists are pushing the boundaries of what is possible, leading to innovations that have the potential to improve human health, protect the environment, and drive economic growth. As research and technology continue to advance, the impact of protein engineering is set to grow, heralding a new era of scientific and technological breakthroughs.
https://www.marketdigits.com/protein-engineering-market-1704808374