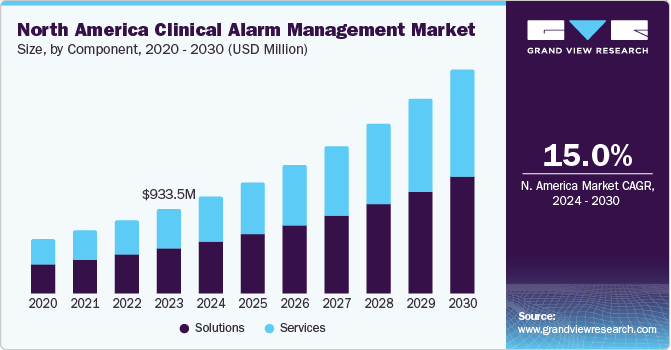
The global clinical alarm management market was estimated at USD 2.13 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 17.0% from 2024 to 2030. Several factors are anticipated to drive this market growth, including the increasing prevalence of alarm fatigue and the necessity for cost-containment strategies in healthcare settings. Additionally, prolonged hospital stays, advancements in technology, and the rising incidence of chronic diseases are expected to further fuel market expansion. Alarm fatigue, in particular, is a pressing issue in healthcare environments, leading to an overwhelming number of non-actionable alarms that can jeopardize patient safety.
Reports such as the Making Health Care Safer (MHCS) from the Agency for Healthcare Research and Quality (AHRQ) underscore the importance of implementing safety culture elements, conducting risk assessments, and providing training to alleviate alarm burdens and enhance overall patient care. False alarms can result in clinicians ignoring true alarms, which are crucial for identifying actual patient issues. This situation drives the demand for effective alarm management solutions.
The advantages of these solutions encompass clinical, operational, and financial aspects. Clinical benefits include improved satisfaction for both patients and their families, better sleep and rest for patients, and a reduction in non-actionable alarms and gaps in alarm protocols. These improvements contribute to enhanced patient safety and potentially fewer missed true positive alarms. Operationally, effective alarm management can lead to decreased alarm fatigue among staff, higher satisfaction levels, reduced burnout, improved productivity, and more efficient use of nursing resources.
Gather more insights about the market drivers, restrains and growth of the Clinical Alarm Management Market
Market Concentration and Characteristics
The market is currently experiencing high growth and operates at an accelerating pace. It is characterized by significant innovation, as the role of alarms in hospital settings continues to evolve. For example, Philips offers various alarm management solutions, including continuous patient monitoring systems, the Patient Information Center iX (PIC iX), and the CareEvent system, reflecting ongoing advancements in the field.
Additionally, there is a high level of merger and acquisition (M&A) activity among leading market players. A notable collaboration occurred in April 2023 when Mobile Heartbeat partnered with Akkadian Labs to integrate the Akkadian Provisioning Manager with MH-CURE. This partnership aims to streamline the provisioning process for Mobile Heartbeat users, enhancing clinical communication and collaboration for healthcare professionals.
The market is also facing increased scrutiny regarding regulations, the number of market participants, capital investments, and the overall buyer landscape. Unlike medical devices, alarm management solutions and services face less stringent regulatory oversight in both developed and developing countries.
In the United States, several guidelines and industry standards for alarm management have been introduced in the past decade, highlighting the growing need for more standardized and validated regulations. The Joint Commission, along with the Association for the Advancement of Medical Instrumentation, the FDA, ECRI, and the American College of Clinical Engineering, has established the current regulations adhered to in the market.
There are limited direct product substitutes available. Given the unique application of these solutions and services in monitoring, identifying, and optimizing alarms in healthcare facilities, no internal or external substitutes currently exist. Consequently, the threat of substitutes in this market is considered low.
Market growth strategies, including strategic agreements, the launch of advanced products, portfolio expansion, and partnerships with key players, are contributing to overall market development. For instance, in March 2022, Koninklijke Philips N.V. announced the FDA 510(k) market authorization for its HIMSS22 Philips Capsule Surveillance solution, aimed at deployment across U.S. healthcare systems. Additionally, Masimo has captured a significant share of the North American market, further underscoring competitive dynamics in this sector.
Order a free sample PDF of the Clinical Alarm Management Market Intelligence Study, published by Grand View Research.